16 Mar EARTH MATTERS: A CASE STUDY IN FOOD REGULATION
 In my last post, I explained that the United States Food and Drug Administration (FDA) is reviewing the application for the commercialization of AquAdvantage salmon — genetically modified salmon. Although the public at large finds confidence in “FDA approved” products, I discussed why perhaps this shouldn’t be the case.
In my last post, I explained that the United States Food and Drug Administration (FDA) is reviewing the application for the commercialization of AquAdvantage salmon — genetically modified salmon. Although the public at large finds confidence in “FDA approved” products, I discussed why perhaps this shouldn’t be the case.
The FDA process creates lack of transparency, lack of expertise and an inadequate review of potential environmental effects. Knowing this, you may appropriately ask, “Why is the FDA the agency reviewing this application?” You’d be asking a question worth explaining.
The early days of recombinant DNA research – the process used for creating genetically modified organisms, GMOs – saw a void in regulation that the scientists themselves felt they had to fill. A committee of experts called for two initiatives 1. A moratorium on more risky experiments and 2. An advisory committee to oversee rDNA research. This advisory council met in Asilomar in 1975 and created a set of research guidelines based on a continuum of hazards, allowing certain types of experiments while precluding other, riskier ones.
Asilomar marked the beginning of US regulation of scientific research on GMOs. It assumed that GMOs would produce benefits, and that any risks should be balanced against that assumption. This justified advancing the research agenda, and kept scientists in control of future GMO policies. Even more importantly, Asilomar focused the regulatory debate on GMO risks on containment – how to keep GMOs in their place – rather than on broader questions of purpose, need, ethics, and cui bono (who benefits).
Containment has proven extremely difficult to control. Pollen spreads to neighboring fields, volunteer crops show up where they are not wanted, mislabeling or poor quality control leads to incidents like StarLink, where GM corn that was not approved for human consumption ended up in taco shells on grocery store shelves. This only makes looking at the broader questions, and thinking about different ways to regulate GMOs, that much more important. But as yet, that has not happened in the US.
In the 1980s, GMOs started moving from labs to fields. The 1980 Supreme Court ruling in Diamond v. Chakrabarty allowed patenting of genetic engineering products and processes. New Reagan-era policies promoted university-industry research partnerships, environmental deregulation, and economic competitiveness that led to a push for more commercial applications of the technology.
Under Reagan, the US government adopted a “science-based” approach to regulation that was premised on the idea that GMOs were “substantially equivalent” to their conventional counterparts despite the introduction of novel genetic material. While some legislators tried to set up new rules and agencies, GMO regulation and management was vested in existing agencies through the 1986 Coordinated Framework for the Regulation of Biotechnology (“Coordinated Framework”). It aims to coordinate regulatory decisions and divide responsibilities among several agencies. Over 15 years later the Coordinated Framework still holds.
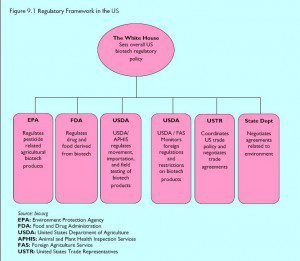 Under the Coordinated Framework, the US Department of Agriculture’s Animal and Plant Health Inspection Service (USDA-APHIS), is responsible for most GM plants. GM crops require APHIS approval for commercial planting, though most are granted “non-regulated” status. Once a plant receives approval – which happens within 30 days of receipt of the file – it is permanently deregulated and the USDA no longer maintains oversight.
Under the Coordinated Framework, the US Department of Agriculture’s Animal and Plant Health Inspection Service (USDA-APHIS), is responsible for most GM plants. GM crops require APHIS approval for commercial planting, though most are granted “non-regulated” status. Once a plant receives approval – which happens within 30 days of receipt of the file – it is permanently deregulated and the USDA no longer maintains oversight.
The FDA, under the Food, Drug, and Cosmetic Act (FDCA), is responsible for regulating food additives, food, and animal drugs. It makes no distinction between most GE food and food produced through other breeding techniques unless there are known allergens in the product. This approach sees GE food as substantially equivalent to non-GE food when their chemical and physical characteristics are similar. It is on this basis, as well, that the FDA does not require labeling for GM products.
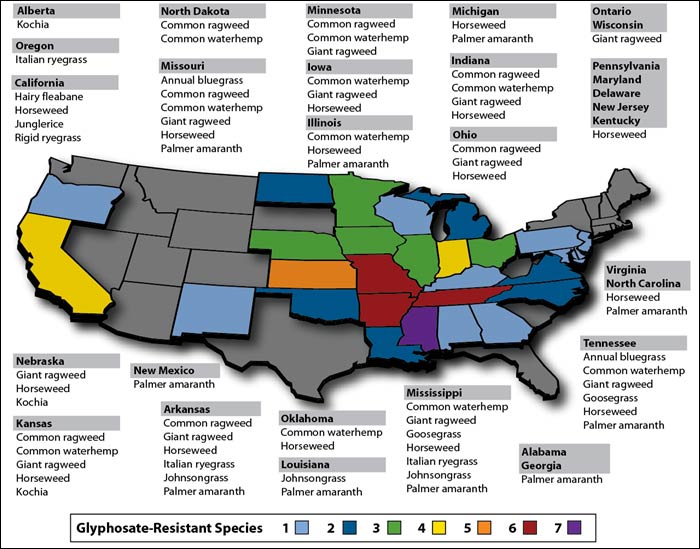
The Environmental Protection Agency (EPA) regulates toxic substances on authority from the Federal Insecticide, Fungicide, and Rodenticide Act, the FDCA, the Toxic Substances Control Act, the Food Quality Protection Act, and the Plant Pest Act. This covers plants engineered to produce their own pesticides or other toxics. The agency is also responsible for insect resistance management plans where plant-producing pesticides might cause insects to evolve pesticide resistance. However, there is little monitoring, and even according to the GM seed companies like Monsanto, there has been widespread non-compliance with these rules, leading extremely rapidly to the pesticide resistance that anti-GM Cassandras warned would occur.
As we have seen with GM fish, this shared responsibility for GMO regulatory oversight often leads to strange or inappropriate divisions of responsibility. Additionally, the Coordinated Framework provides no way to determine whether GMOs, once released, have caused harm. Finally, without labels on GM foods, there is no way to trace adverse health reactions, and no way for consumers to decide whether to purchase GM products other than by buying organic products, which, despite efforts by industry to allow GMOs to be considered organic, to date may not contain any genetically modified ingredients.
What might some solutions be, and how could they be implemented? I will explore these questions in a later posting.


Sorry, the comment form is closed at this time.